Previous: Introduction Next: Model description and experiments Up: Ext. Abst.
Chemistry of ![]() in the middle atmosphere
in the middle atmosphere
Mesospheric loss of ![]() has been evaluated by different authors: specific global lifetimes
of 13 500 and 4200 years respectively were found when assuming
either Lyman-
has been evaluated by different authors: specific global lifetimes
of 13 500 and 4200 years respectively were found when assuming
either Lyman-![]() or a dissociative electron attachment to destroy the molecule
(Ravishankara et al., 1993). When assuming photodissociation by UV radiation with
a wavelength of less than 240 nm, a lifetime of 1000 years was
estimated (Ko et al., 1993). In 2-D model calculations even an atmospheric lifetime
as low as 800 years was found (Morris et al., 1995). For our study we use a simplified chemistry scheme (Reddmann et al., 2000), but besides electron attachment and photodissociation
we also consider reactions of
or a dissociative electron attachment to destroy the molecule
(Ravishankara et al., 1993). When assuming photodissociation by UV radiation with
a wavelength of less than 240 nm, a lifetime of 1000 years was
estimated (Ko et al., 1993). In 2-D model calculations even an atmospheric lifetime
as low as 800 years was found (Morris et al., 1995). For our study we use a simplified chemistry scheme (Reddmann et al., 2000), but besides electron attachment and photodissociation
we also consider reactions of ![]() and evaluate uncertainties of reaction rate constants by comparing
several chemical scenarios.
and evaluate uncertainties of reaction rate constants by comparing
several chemical scenarios.
The reaction of ![]() in the mesosphere can be described by the following scheme which
extends that given by Odom et al. (1975) by adding the reactions of
in the mesosphere can be described by the following scheme which
extends that given by Odom et al. (1975) by adding the reactions of ![]() :
:
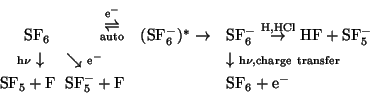
Reaction rates used are given in 1, note the variants for reaction
R8. Details of the chemistry scheme are given in Reddmann et al. (2000).
| Id | Reaction | Total rate constant in |
Rate at 60 km in |
Remarks |
| R1 | destructive | |||
| R2 | 270 | |||
| R2a | destructive branch of R2, branching fraction |
|||
| R5 | 0.19 | |||
| R6 | ||||
| R3 | 0.3 | Photodetachment | ||
| R4 | 0.21 | destructive | ||
| R7 | 1.5 | destructive | ||
| R8a | 0.032 | |||
| R8b | 1.2 |
![\includegraphics*[width=85mm, trim=0 0 0 0]{jgr9904.eps}](img26.gif) |
The result of the chemistry scheme of ![]() depends on the assumptions made for some reaction rates and electron
density. To test the influence of various parameters we choose
several combinations in the model runs. The electron density profile
is represented by four versions to account for the uncertainties
especially in the mean electron energy and to test the influence
of diurnal and seasonal variation, see Fig. 1. All scenarios tested
are given in Table 2.
depends on the assumptions made for some reaction rates and electron
density. To test the influence of various parameters we choose
several combinations in the model runs. The electron density profile
is represented by four versions to account for the uncertainties
especially in the mean electron energy and to test the influence
of diurnal and seasonal variation, see Fig. 1. All scenarios tested
are given in Table 2.
| Notation | Description | Electron profile |
| S1 | direct loss by electron attachment (R2) | B |
| S2 | S1 + R3 + R4 + R5 | B |
| S3 | S2 + R7 + R8a | B |
| S4 | as S3, but R8b | B |
| S5 | as S3 | B1 |
| S6 | as S3 + R6 | B |
| S7 | as S4 | A |
| S8 | as S7 + cosmic ray ionisation | A1 |
| See text for versions of electron profiles, | ||
| see Table 1 for the identification of reactions. | ||