Previous: Stratospheric ozone Next: Nitrous oxide Up: Ext. Abst.
Water vapour
Water vapour is both the most important greenhouse gas, accounting for 60 % of the total radiative heating, and a key factor for stratospheric ozone depletion through its role of condensing into ice clouds which boosts heterogeneous chemistry (Kirk-Davidoff, 1999). This contrasts with our poor understanding of water in the atmosphere which adds the greatest uncertainty to present cli mate models. To date it is unclear how much of the observed increase in stratospheric water vapour is a consequence of enhanced methane oxidation and how much results from enhanced vertical transport of humid air (Forster, 1999). Water vapour from each of these two major sources has a significantly different iso topic composi tion (Irion, 1996a). Simultaneous measurement of water vapour VMRs and their isotopic composition may enable us to resolve the current ambiguity regarding the sources of stratospheric water vapour increases and to quantify the exchange of water vapour across the topopause. Our observations may also provide valuable input to recent models that include isotopic information on water vapour (Hoffman et al, 1998).
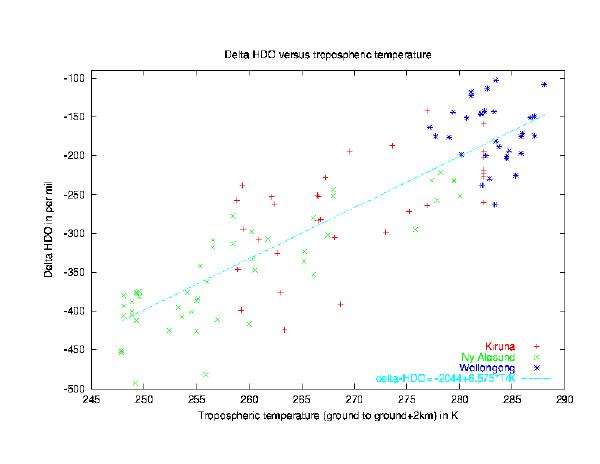
A clear correlation of the delta-D values with the ambient temperature is found that is consistent for three sites studied. As expected theoretically, heavy isotopes are removed preferentially by condensation and subsequent precipitation due to the differences in the lower ground state energies and vapour pressures. Cold and dry air masses are thus stronger depleted in Deuterium. Unlike the case of ozone, water vapour isotopic fractionation is fully understood by classical mass-dependent fractionation processes that occur with every phase transition. The first comprehensive description given by Daansgard (1953) is still valid today and explains all aspects in a quantitatively correct way.
|
|
|
Figure 2: This figure shows the depletion in Deuterium in tropospheric
water vapour relative to Standard Mean Ocean Water (SMOW) as a
function of the mean ambient temperature from ground to ground+2km.
About 90 % of all water vapour molecules are located in the lowermost
2 km making this temperature a good approximation for our observations.
The depletion is expressed in the ?-notation with ?D defined as
?D =(([D/H]sample/[D/H]reference)-1)·1000
The measurements reported are taken from 3 sites: Wollongong (35°S), Kiruna (68°N) and Ny-Alesund (79°N). The symbols represent individual measurements unbiased in
seasons.
Although the VMR profiles both of normal H2O and HDO that we obtained (poster figure not included here) look quite reasonable, the delta-D values raise questions about their trustworthyness. It is noted that the water vapour VMR profiles of normal H216O drop more rapidly in the 2 to 5 km region than suggested by VMRs calculated from radio sonde relative humidity data. The underestimation of H in the 2 to 4 km region results in much too high D/H ratios and the resulting delta-D values are completely unreasonable between 2 and 4 km. The spectroscopic data of water vapour in HITRAN96 (in particular the pressure broadening coefficients which determine the altitdue distribution retrieved) are known to be of limited quality. Bob Toth has recently remeasured large regions in the infrared consistently for H2O and HDO, though unfortunately the lines used in the present study were not included (yet). However, the improvements in the regions covered thus far are most encouraging and we are confident that both the use of alternative lines in those regions and the ongoing work by Toth and co-workers will allow us to overcome these problems in the near future. Please note, that the isotopic ratios calculated for the total column amounts are little affected by this problem and that results reported for FTIR total columns are in good agreement with theoretical expectations and in-situ measurements.
Previous: Stratospheric ozone Next: Nitrous oxide Up: Ext. Abst.