State University of New York, Stony Brook, N.Y., 11794, U.S.A.
FIGURES
Abstract
1. Introduction:
Various theoretical and experimental studies have been made in recent years to determine seasonal rates of descent within the polar vortices during winter. Vortex descent has the important effect of altering profiles of mixing ratio versus altitude for various species, and therefore altering the speed and equilibrium points for chemical reactions between species, due to their (frequently) strong dependence on pressure and temperature. Moreover, descent of HNO3, H2O, and H2SO4 into the very low temperature region that forms by mid winter in the 15-25 km altitude range leads to large scale gravitational removal of these species from the lower stratosphere, following their condensation into Polar Stratospheric Cloud (PSC) particles. This has an even more profound effect on stratospheric vortex chemistry.
An early measure of descent in the lower stratosphere was made by Parrish et al., [1988] who observed a dramatic flattening of the mixing ratio profile of N2O over McMurdo Station, Antarctica, by the end of the polar winter, when compared with earlier summer measurements. ER-2 aircraft, ATMOS, and several UARS instruments have added a number of other data sets from which to derive vortex descent rates, though these do not extend beyond ~80º S, and frequently do not reach that far. The edge region of the vortex is generally thought to descent more rapidly than the core. Quasi-inert tracers such as N2O and CH4 are usual chosen for experimental descent measurement.
Backmeister, et al. [1995], Strahan, et al. [1996], and Allen, et al. [2000], among others, have variously noted that different tracers yield different apparent descent rates, and have explained this as a result of mixing across the vortex boundary in the presence of different gradients for different species (see below). To date, these investigations have been limited to analysis of data between ~60-80º S. In what follows, we show that similar results hold true to the center of the vortex core, and not merely its outer regions, and we provide new views of vortex descent.
Diabatic descent has been investigated theoretically by several groups. For instance, Rosenfield et al. [1994] have estimated diabatic descent rates from various altitudes within the polar vortices by calculating diabatic cooling from UKMO assimilated temperature fields for 1992 and 1993, considering the vortices as isolated systems without cross-boundary transport. More recently, Rosenfield et al. [2000] have recalculated polar diabatic descent rates using back trajectory calculations on air parcels over periods up to seven months in an effort to determine average origins and thermal history of air reaching the vortex core by mid-winter. They conclude that descent predicted by this technique is always slower than descent rates calculated on the basis of an ‘isolated’ vortex, because horizontal motion and variation in heating (cooling) rates with latitude and longitude reduce the time-integrated rates of cooling. The use of multi-month back trajectories, even though used only to establish average origins and motions, may bias results however, and it is probably best to take the original [1994] and more recent [2000] calculations of Rosenfield et al. to represent approximate extremes within which real vortex behavior may lie.
Working with ground-based mm-wave measurements of O3 taken for most of a year over the South Pole in 1993, we investigated the use of O3 as a tracer of stratospheric vortex core descent in a paper by Cheng et al., [1996]. Although O3 is essentially inert chemically in the middle-atmospheric winter darkness, it was found that descent traced by constant mixing ratio contours of O3 versus time showed physically incorrect slower descent in the upper stratosphere than in the middle stratosphere. We revisit that observation here, extending the observations to the austral winter of 1999 and to apparent descent rates of HNO3 as well as O3 and N2O versus altitude, inferred from profiles retrieved by deconvolution from pressure-broadened mm-wave emission lines.
2. Experimental descent rates inferred from N2O, O3, and HNO3:
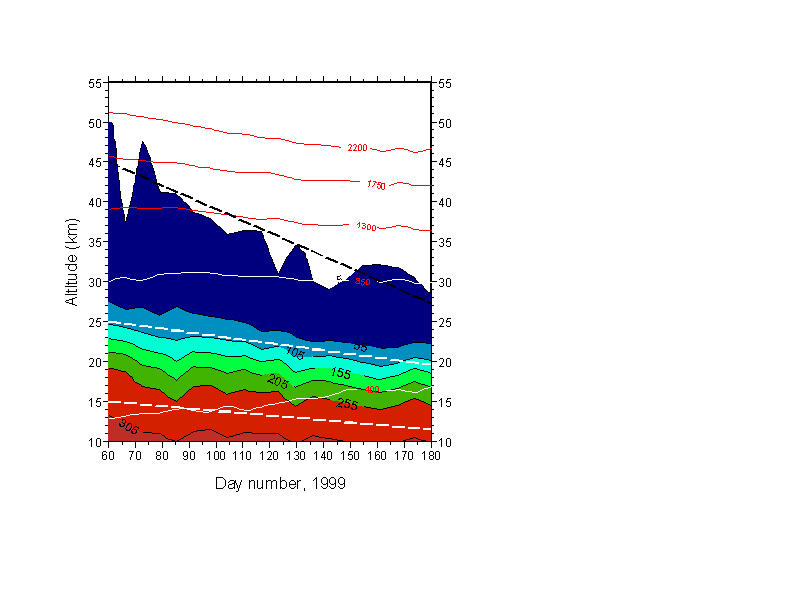
Starting with N2O as an inert tracer at lower altitudes, Fig. 1 shows that mixing ratio contours over the four month period from ~day 60 (March 1) to ~day 180 (June 29) of 1999 show remarkably constant descent in the range 10-25 km. Note that descent starts in the vortex well before sunset (~March 21 = day 80). The linear descent line starting at 45 km has been given the same final altitude as achieved on day 180 by the descent calculated by Rosenfield et al. [1994] during the same period. All other sample descent lines are deduced from the data only. Numerical equivalents are given in Table 1. The overall agreement is very good, although the "45 km" is merely the minimum detection level, and not a very secure day-by-day measurement. Note that the isentropic surfaces represented by constant potential temperature are in all cases cut by the diabatic descent indicated by mixing ratio contours.

Figure 1: Constant mixing ratio contours for N2O measured over the South Pole, from March 1 - June 29, 1999. Data from 1993 and 1995 displays very similar behavior. Sunset occurs at ground level on day 81. Fine lines indicate various potential temperature isopleths. Descent is clearly non-adiabatic. For simplicity, only linear descent rates are indicated from start to end of this period (heavy dashed lines). That starting at 45 km is drawn to exhibit only the total descent for this period calculated by Rosenfield, et al. [1994]. True descent is non-linear, and fastest at high altitudes. See text for further comments.
Fig. 2 shows ozone mixing ratio contours over days 60-180. During darkness, (> day 81) stratospheric ozone is nearly inert in the middle and upper stratosphere, yet the behavior appears markedly different from that of N2O, or the descent rates of Rosenfield et al [1994], shown as heavy dashed curved lines. Descent appears very weak , and seems to accelerate, rather than slowing, as air moves downward. The nominal linear descent lines (heavy white dashes) approximating the behavior of selected mixing ratio contours are much shallower in the upper stratosphere (e.g. starting at 50 km) and rather steeper in the middle stratosphere (e.g., starting at 35 km) than the theoretical descent rates. There appears to be no descent below ~17-18 km, again in contrast to the behavior of N2O.
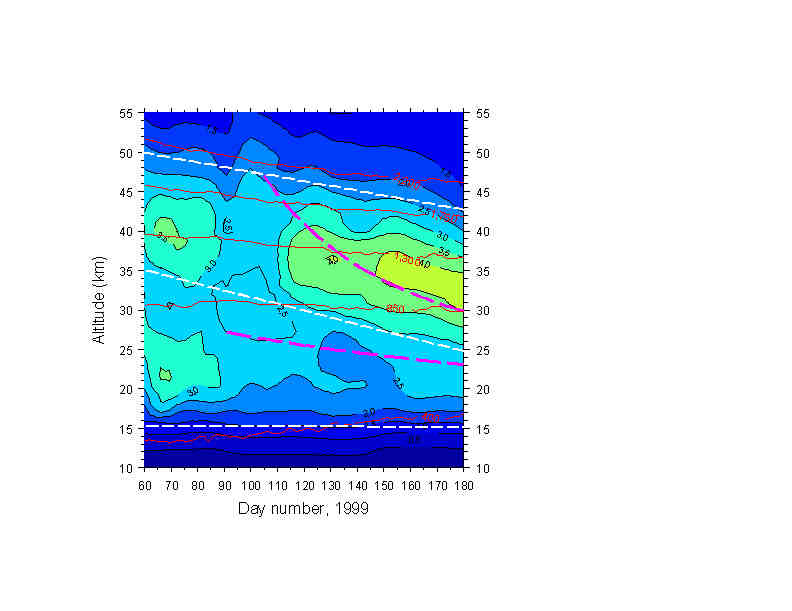
Figure 2: O3 contours for the same period, 1999, as Figure 1. Heavy curved dashed lines show descent rates from Rosenfield, et al., [1994], and are clearly not in agreement with descending O3 isopleths, which appear to show unphysically slow descent at high altitudes.
Fig. 3 shows mixing ratio contours for HNO3 for days 60-180. Note the change of vertical scale. No HNO3 data were taken over the blank period from days 107-117. HNO3 is photochemically active before sunset, and increases strongly in the weeks after sunset, at altitude-dependent rates, as NOx ® N2O5® HNO3. The surge of formation beginning after ~day 100 is eventually supplanted by rapid condensation, again altitude dependent, as HNO3 condenses into PSCs below 25 km, while new formation takes place above 25 km after ~day 150. Although this chemical activity should render HNO3 a very poor tracer, "descent" seems visible in the contour trends before and shortly following sunset, and for comparison I have indicated linear descent rates (heavy dashed lines) from selected altitudes equal to those determined from N2O, over days 60-100. It appears that during this period, downward transport still dominates the vertical distribution of HNO3. By contrast, the period ~day 120-140 shows no discernible downtrends in contours (sample lines at 30, 25, 20 km) as heterogeneous conversion of N2O5 and other altitude and time-dependent chemistry take control.
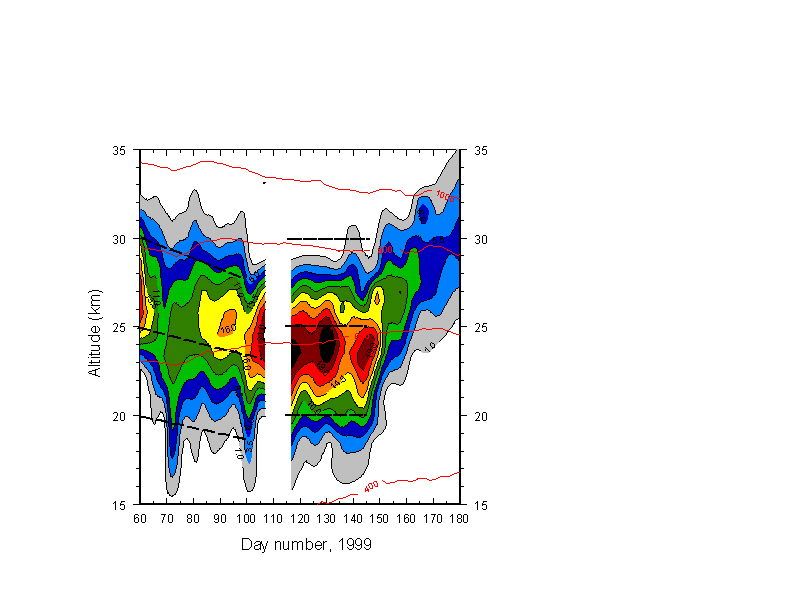
Figure 3: As in Figures 1 and 2, but for HNO3. Subjective indications for descent of mixing ratio isopleths has been divided into two segments. Prior to sunset HNO3 is in approximate photolytic equilibrium in the stratosphere. After sunset, HNO3 begins a rapid, altitude-dependent increase as NOy is shifted into HNO3. Days 120-145 show little if any indication of isopleth descent, in contrast with the inert tracer N2O (Fig. 1). After ~day 145, condensation rapidly removes HNO3 from the lower stratosphere, while new molecules form at higher altitudes.
Fig. 4 covers a somewhat later period, where the full range of HNO3 formation can be seen in the mid-to-upper stratosphere. Here, I have shifted to 1993 data, due to another unfortunate interruption of data-taking during this period in 1999. The heavy dashed black line again represent the descent calculations of Rosenfield et al. [1994] for the times and altitudes indicated. It looks persuasively like the descent marked by HNO3, (ignoring a probable dynamical event around day 200) if one traces the descent of the peak mixing ratio, rather than constant contours, realizing that an increasing amount of nitric acid is being formed via ion-cluster enhanced chemistry as vortex air continues to descend, with formation efficiency probably increasing when temperatures become cold enough for sulfate condensation at ~35-40 km [de Zafra and Smyshlyaev, in preparation, 2000]. (The empty region below 25 km has been cleared of gas-phase HNO3 by PSC formation. The upper boundary of this region descends faster than the calculated descent rate (not shown) after ~day 210 as the temperature boundary exceeding the PSC evaporation threshold descends and small or new PSCs, which have not yet gravitationally settled, evaporate and return HNO3 to the gas phase [McDonald et al., 2000].)
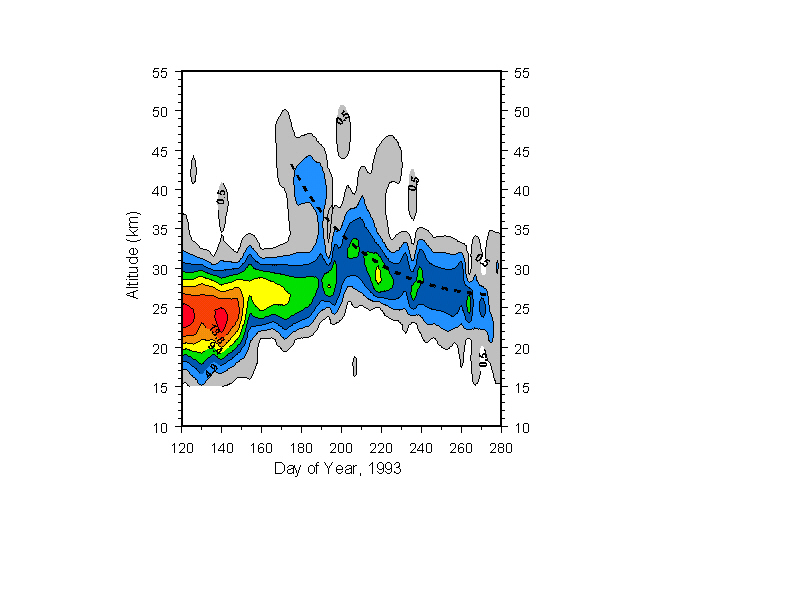
Figure 4: HNO3 for days 120-280, 1993. Formation of high-altitude HNO3 appears to be accompanied by rapid downward transport in general agreement with Rosenfield, et al. [1994], shown by the heavy dashed curve. HNO3 increases in mixing ratio as air is transported downward due to continued formation, so the track of peak mixiing ratio, rather than constant mixing ratio, is followed. See text.
Table 1 gives numerical estimates for overall linear descent rates inferred from descent of mixing ratio isopleths for N2O, O3, and HNO3, contrasted with the overall diabatic descent calculated by Rosenfield et al. [1994] for an isolated Antarctic vortex, also re-expressed as a linear rate. In reality, true air descent is of course faster at higher altitudes, and slows as descent progresses. The time period is between days 60 and 180 for N2O and O3, and between days 60 and 140 (to avoid the PSC condensation period) for HNO3, further divided into two sub-periods, as described above.
Agreement between N2O and Rosenfield et al. [1994] is surprisingly good for all altitudes covered except 20 km, where subsidence becomes somewhat uncertain. Agreement for O3 is, conversely, very poor. HNO3 is a separate case, due to its chemical activity both before and after sunset. Nevertheless, its descent from high altitudes in mid-winter (Fig. 4, graphed but not tabulated) appears to be in reasonable agreement with Rosenfield, et al. , as is the more speculatively determined descent over days 60-100
Why does upper stratospheric O3 appear to descend more slowly than mid-stratospheric O3, and in neither case approach agreement with theory, in contrast to N2O? Why does mid-to-upper stratospheric HNO3 appear to show a much more rapid descent than O3 within the same altitude range during mid to late winter, when both species, once formed, are supposed to be chemically inert in the winter darkness? Accepting the later premise, one must invoke a dynamical explanation [e.g., Backmeister et al., 1995, Strahan et al., 1996]. Different species may be expected to show different apparent descent rates if they have different mixing ratio gradients across the vortex boundary and if cross-boundary transport is significant. An extreme case is reached (Fig. 5) when considering species having opposite gradients, so that cross-boundary transport can act to decrease or increase the apparent rate of descent, depending on the sign of the gradient. The establishment of gradients, and their magnitudes, is time-dependent, however, so that the picture becomes more complicated -for instance, near the time when the vortex is gathering strength. In any event, one might expect to see significant contrasts in apparent vertical transport that depend, in fact, on the average cross-boundary transport relative to the rate of vortex descent, all of which typically varies with time.
|
|
|
|
O3 Apparent rate m/day |
|
|
|
50 45 40 |
n.a. 146 117 |
* (146) * |
58 ±16 - 63 ±17 |
* * * |
* * * |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
† for days 60-95: values chosen to match N2O, as shown in Fig. 3
‡ for days 120-140.
n.a. = values not available.
( ) = speculative determination; descent line in Fig. 1 set to equal the model calculations.
[ ] = speculative determination; descent lines in Fig. 3 set to equal the N2O measurements.
* = mixing ratios too small to track accurately
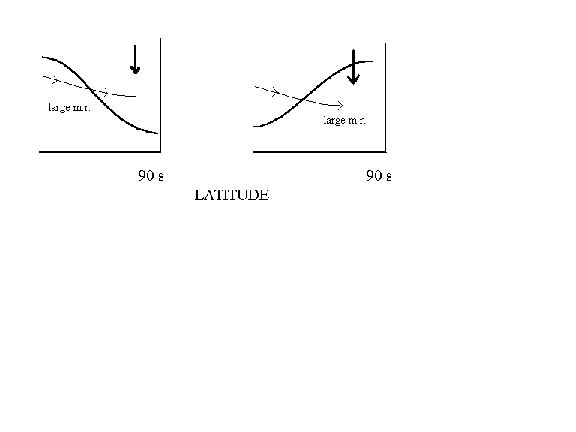
Figure 5: Schematic of a constant mixing ratio isopleth versus latitude (heavy curved line) for examples where gradient is negative (left panel) or positive (right) moving into the vortex. Cross-boundary air motion is shown by light line. Assume that mixing ratio decreases with altitude in the height range depicted. In the left panel, cross-boundary transport tends to sustain larger mixing ratios vs. altitude as air descends, making vertical descent appear slower than actual. The reverse is true for the right-hand panel.
Conclusions from Section 2. Different tracers (e.g., N2O, O3), even during times when thought to be chemically non-active, can give quite different apparent rates of downward transport at the core of the Antarctic winter vortex as well as nearer the vortex edge, for reasons associated with cross-boundary transport. A knowledge of cross-boundary tracer gradients, coupled with simultaneous apparent descent rates for several tracers, should give a better measure of mean cross-boundary transport (as a function of altitude and time) via the adjustment of transport equations to simultaneously satisfy the observed differences in apparent descent.
3. Removal of Stratospheric NOy during the Antarctic Winter:
Our measurements of HNO3 from its peak in mid-fall to its mid-winter minimum (April-May to July-August) show that the column density of HNO3 decreases by about 2x1016 molecules/cm2 during this period, and that very little returns to the gas phase as temperatures rise above the PSC maxima. The latter observation indicates that gravitational settling has removed most PSCs from the lower stratosphere by this time. UARS/MLS satellite mapping of HNO3 depletion show that the area involved is typically at least as large as the continental area of Antarctica. A simple calculation thus yields a value of at least 3x108 kg of NOy removed from the stratosphere over Antarctica each winter, given the rather accurate assumption that nearly all stratospheric NOy is in the form of HNO3 by late fall or winter. This estimate does not include additional smaller quantities of NOy (primarily NO2) which undergo transformation to HNO3 in the mid-to-upper stratosphere in winter, and are transported downward far enough to encounter PSC formation temperatures and condense out. I estimate that at perhaps an additional 0.3x108 kg.
The overall amount of NOy removal has probably been increasing in recent decades, as well-documented increases in stratospheric CO2 and water vapor lead to faster radiative cooling, lower winter temperatures, and an earlier, longer period of PSC formation in the lower stratosphere. Greatly diminished ozone also delays springtime warming, leading to longer gravitational settling times that continue to move small as well as larger HNO3-bearing PSCs out of the lower stratosphere.
Acknowledgments: This work was supported by the National Science Foundation (U.S.) under Grant No. OPP 9705667, and by NASA under grant NAG 54071. I thank Curt Trimble for dedication over and beyond the call of duty to the task of year-long data-taking at the South Pole, and to Giovanni, Muscari, Anca Nemuc, America Cañas, and Chad Bender for assistance in data reduction and other tasks.
References:
Allen, D.R., et al., Antarctic polar descent and planetary wave activity observed in ISAMS CO from April to July a992, Geophys. Res. Letters, 27, 665-668, 2000.
Backmeister, J.T., et al., Descent of long-lived trace gases in the winter polar vortex, J. Geophys. Res., 100, 11,669-11,684, 1995.
Cheng, D., R.L. de Zafra, C. Trimble, Millimeter-wave spectroscoic measurements over the South Pole, 2. An 11-month cycle of stratospheric ozone observations during 1993-94, J. Geophys. Res., 101, 6781-6793, 1996.
McDonald, M., R. L. de Zafra, and G. Muscari, Millimeter wave spectroscopic measurements over the South Pole 5: Morphology and evolution of HNO3 vertical distribution, 1993 versus 1995, J. Geophys. Res. 105, 17,739-17,750, 2000.
Parrish, A., R.L. de Zafra, M. Jaramillo, B. Connor, P.M. Solomon, J.W. Barett, Extremely low N2O concentrations in the springtime stratosphere at McMurdo station, Antarctica, Nature, 332, 53-55, 1988.
Rosenfield, J. E., P. A. Newman, and M. R. Schoeberl, Computations of diabatic descent in the stratospheric polar vortex, J. Geophys. Res., 99, 16,677-16,689, 1994.
Rosenfield, J. E., and Schoeberl, M. R., Studies of fall to spring Arctic and Antarctic polar vortex descent, Proceedings of the Quadrennial Ozone Symposium, Sapporo, 2000; pgs 615-616, 2000.
Strahan, S.E., J.E. Nielsen, and M. C. Cerniglia, Long-lived tracer transport in the Antarctic stratosphere, J. Geophys. Res. 101, 26,615-26,629, 1996.
Back to
| Session 1 : Stratospheric Processes and their Role in Climate | Session 2 : Stratospheric Indicators of Climate Change |
| Session 3 : Modelling and Diagnosis of Stratospheric Effects on Climate | Session 4 : UV Observations and Modelling |
| AuthorData | |
| Home Page | |