Previous: Experimental details Next: Conclusion Up: Ext. Abst.
Results and discussion
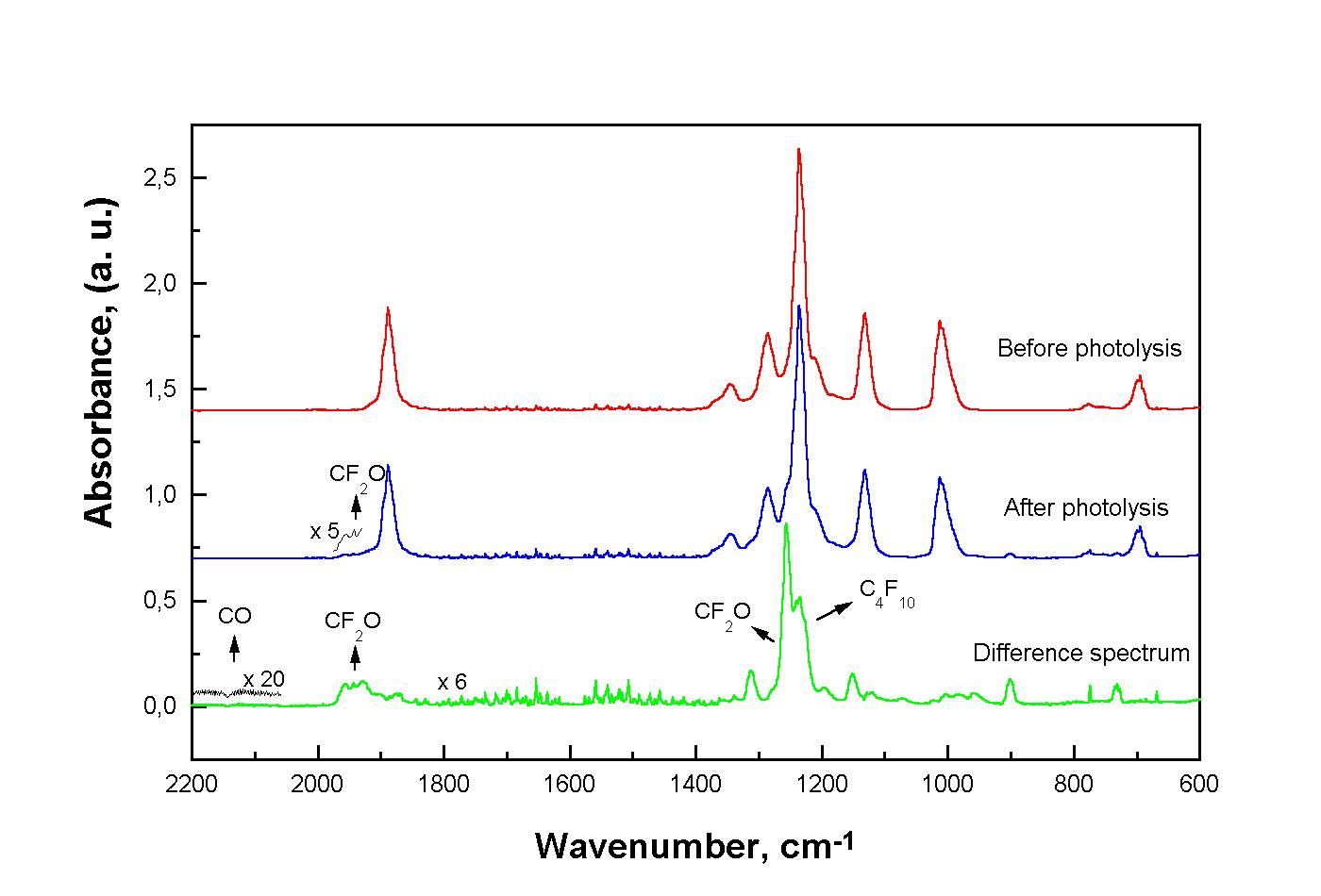
The comparison between the photolysis rate of CF3CF2C(O)F alone and with either c-C6H12 or (FCO)2 was performed using the setup described above. When CF3CF2C(O)F alone was photolized, the products formed were C4F10, CO and CF2O (Fig. 1), in agreement with the results of Weibel et al.3 These products could be explained taking into account that the C-C bond breaking gives CF3CF2 and FCO radicals:
CF3CF2C(O)F ® CF3CF2+ FCO (1)
The CF3CF2C(O)F and CF2O quantification wereperformed using calibration curve for each pure substance.

Fig. 1: IR spectra obtained for the photolysis of pure CF3CF2COF at 254 nm.
The experiments carried out in the presence of c-C6H12 gave CF3CF2H andHFCO as products. In these experimental runs it was not observed C4F10 or CF2O formation.This fact indicates that all the CF3CF2 and FCO radicals formed reacted with the c-C6H12 molecules:
CF3CF2 + c-C6H12 ® CF3CF2H + c-C6H11 (2)
FCO + c-C6H12 ® HFCO + c-C6H11 (3)
When comparing the CF3CF2C(O)F photolysis rate alone and in the presence of c-C6H12(Fig. 2), it can be seen that the CF3CF2C(O)F amount reacted is greater in the second case. This observation suggests the CF3CF2 and FCO radicals react among them in a recombination reaction when there is no other gas added:
CF3CF2 + FCO ® CF3CF2C(O)F (-1)
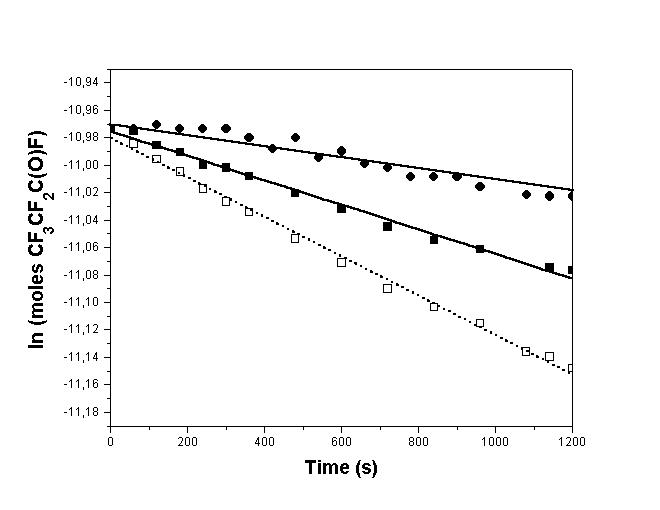
Fig. 2: Plot of ln(moles CF3CF2COF) vs. photolysis time. (", with (FCO)2 added; $, alone; %, with c-C6H12 added). The lines correspond to the least squares fitting to the experimental points
To corroborate this possibility we carried out a new CF3CF2C(O)F photolysis in the FCO radicals presence using a good FCO source such as oxalyl fluoride, (FCO)2. In this case, the C4F10 formed as well as the reactant consumed are smaller than when CF3CF2C(O)F is photolysed alone. In Fig. 2 it can be seen the photolysis rate and in Fig. 3 are shown the spectra obtained in this new condition.
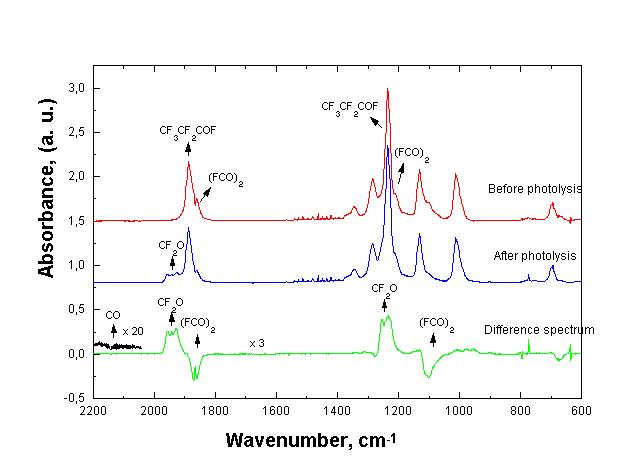
Fig. 3: IR spectra obtained for the photolysis of CF3CF2COF in the presence of (FCO)2 at 254 nm.
In accordance with the results presented previously it is clear that the presence of an effective radical scavenger, able to trap radicals that participate in reactions (2) and (3), allows the reaction photolysis rate determination (1).
Two photolytic experiments were performed with initial CF3CF2C(O)F concentration of 4 x 1016 molecule cm-3 in absence and in presence of c-C6H12 (90 torr). Photolysis time was 1200 s, to maintain low conversions. A comparison of the CF3CF2C(O)F concentration variation between the two runs is presented in Fig. 2. Note that the CF3CF2C(O)F disappearance is faster in the last case. From its slope (dotted line in Fig. 2), we obtain the CF3CF2C(O)F photolysis rate value of (1.4 ± 0.1)x 10-4 s-1.
The mechanism proposed after the C-C bond rupture, when the photolysis is carried out in c-C6H12 absence, is:
CF3CF2 + FCO ® CF3CF2C(O)F (-1)
CF3CF2 + CF3CF2 ® C4F10 (3)
FCO + FCO ® CF2O +CO (4)
The k3 and k4 rate constants were obtained from literature. In the case of k4, there are four values reported but only the work of Behr et al.5 was carried out at pressures comparable to those used in our experiments, (although independent) so we took the value (1.7 ± 0.3) x 10-11 cm3 molecule-1 s-1. For k3the value informed is 2.85 x 10-12 cm3 molecule-1 s-1.6
With the mechanism just proposed, we simulated the time variation of the CF3CF2C(O)F and CF2Oconcentration. The experimental and simulation results are shown in Fig. 4. The best fiitting of the k-1 value gives (6.8 ± 0.8)x 10-12 cm3 molecule-1 s-1. This value of the recombination rate constant also explains why, in the CF3CF2C(O)F photolysis in presence of oxalylfluoride, the C4F10 concentration drops so efficiently and the CF3CF2C(O)F concentration decreases much less than when it is photolysed alone.
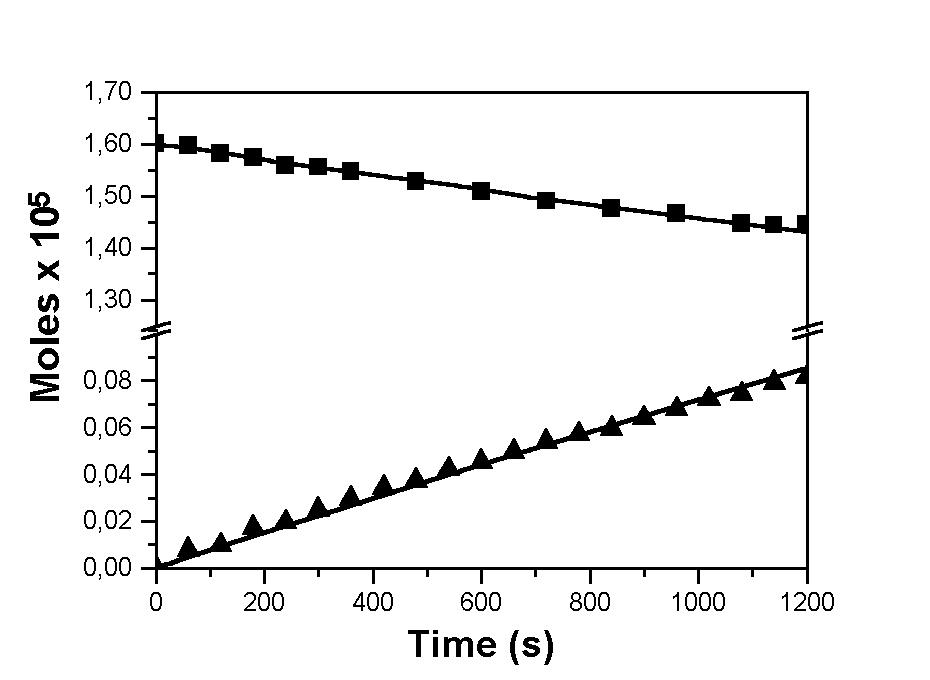
Fig. 4: Fitting to the actual concentration of species ($, CF3CF2COF; Å, CF2O) when the value assumed by k-1 in the simulation is 6.8 ± 0.8)x 10-12 cm3 molecule-1 s-1.