Previous: Introduction Next: Results and discussion Up: Ext. Abst.
Experimental details
Commercially available samples of perfluoropropyl fluoride (CF3CF2C(O)F) (P.C.R. Research Chemicals) were trap-to-trap distilled in vacuum before use and their purity was checked by IR spectroscopy. Cyclohexane (98%) was used as received. Oxygen was condensed by flowing O2 at atmospheric pressure through a trap immersed in liquid air. It was then pumped under vacuum several times and transferred to a glass bulb whilst the trap was still immersed in liquid air. Oxalylfluoride (FCO)2 was synthetized by fluorination of oxalylchloride with NaF in sulfolane as solvent and purified by trap-to-trap distillation4.
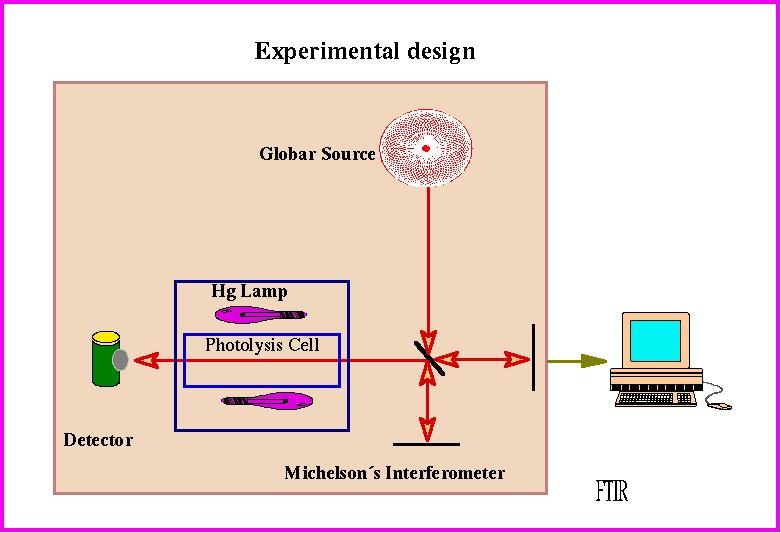
Reactants and products were manipulated in a conventional high-vacuum system. The photolyses were carried out using low pressure Hg lamps surrounding a quartz cell fitted with KCl windows which was located in the optical path of a Fourier transform IR (FTIR) spectrometer which was used to follow the evolution of the reaction with time ( see Scheme 1).

Scheme 1