 |
Stratospheric Processes And their Role in Climate
|
||||||||
| Home | Initiatives | Organisation | Publications | Meetings | Acronyms and Abbreviations | Useful Links |
![]()
 |
Stratospheric Processes And their Role in Climate
|
||||||||
| Home | Initiatives | Organisation | Publications | Meetings | Acronyms and Abbreviations | Useful Links |
![]()
Peroxy radicals are central to ozone photochemistry in the troposphere and lower stratosphere. The simplest of the family, HO2, interconverts readily with OH on a time scale of seconds.
HO2 + NO Æ OH + NO2
OH + CO (+ O2) Æ HO2 + CO2
Organic peroxy radicals are formed in the oxidation of hydrocarbons and other organic molecules. For example, methane reacts to form the methyl peroxy radical, CH3O2, which reacts rapidly with NO to give the methoxy radical.
OH + CH4 Æ H2O + CH3
CH3 + O2 + M Æ CH3O2 + M
CH3O2 + NO Æ CH3O + NO2
The reactions of peroxy radicals with NO are the critical steps in the photochemical formation of ozone, since the NO2 generated photodissociates at wavelengths which are available throughout the troposphere (l > 300 nm).
NO2 + hn Æ NO + O
O + O2 + M Æ O3 + M
This combination of a hydrocarbon "fuel", NO "catalyst", and sun light lead to photochemical ozone production when molecular oxygen cannot be photodissociated (Figure 1).
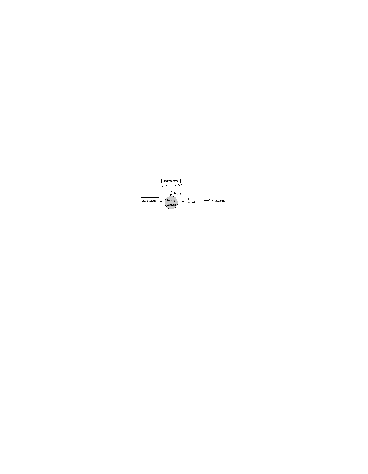
Figure 1. A simplified scheme that shows the pathway for the photochemical production of ozone in the troposphere. The key role of peroxy radical in ozone production is exemplified.
In NOx-poor regions of the atmosphere, reactions between peroxy radicals become more important. Of particular interest are those between peroxy radicals and HO2, which usually lead to the formation of a stable peroxide product, such as methyl hydroperoxide.
CH3O2 + HO2 Æ CH3OOH + O2
Further physical removal of this peroxide, e.g. by rain out, can lead to permanent removal of the radicals from the atmosphere, and hence a change in the oxidising potential of the region. The interactions centred on peroxy radicals, including the routes for ozone and free radical formations are shown in Figure 2.
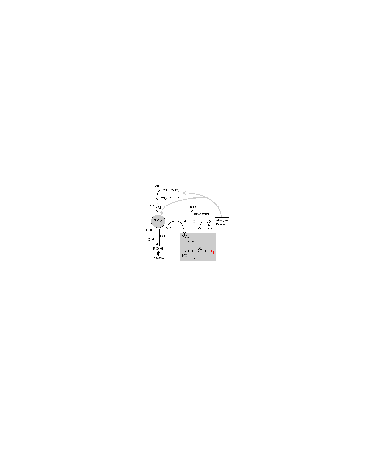
Figure 2. A schematic of the chemical processes involved in the oxidation of hydrocarbons in the atmosphere in the presence of nitrogen oxides that leads to the production of ozone, other free radicals, and stable molecules.
Many of the products formed in peroxy radical reactions (peroxides, carbonyls) can photodissociate to produce free radicals. For example, it has recently been proposed that acetone can compete with ozone photolysis as a source of HOx in the upper troposphere and lower stratosphere. The destruction of acetone leads to the formation of acetonyl peroxy radicals, CH3C(O)CH2O2, in the case of OH attack, or acetyl peroxy, CH3C(O)O2, and methyl peroxy radicals if photolysis occurs.
CH3C(O)CH3 + OH (+ O2) Æ CH3C(O)CH2O2 + H2O
CH3C(O)CH3 + hn (+ 2 O2) Æ CH3C(O)O2 + CH3O2
In light of the recent modelling activity regarding acetone and the organic hydroperoxides, it was considered timely to evaluate what is known about the reactions of the simple peroxy radicals with each other and with HO2, NO and NO2.
Reactions of peroxy radicals have been studied in the laboratory for over 20 years now. The studies have not always been definitive, since good, direct ways to detect the radicals have been slow in development. Almost all the studies have used ultraviolet absorption spectroscopy, which is not particularly sensitive, or specific to a given radical. The studies are further complicated by the fact that the products of the reactions are often other peroxy radicals which absorb in the same spectral region and undergo reactions with the peroxy radical of interest. Add to this the necessity to generate and detect two radicals simultaneously, and it is clear that great care needs to be taken in planning and executing an experiment. Furthermore, previous data was not always reinterpreted in light of new information. With all these things in mind, an evaluation of the chemistry of some atmospherically important peroxy radicals has been prepared under the SPARC and IGAC tasks related to laboratory studies. Participants were G.S. Tyndall (NCAR, chair), R.A. Cox (Cambridge, IGAC co-ordinator for the Laboratory Studies joint project), R. Lesclaux (Bordeaux), G.K. Moortgat (MPI Mainz), M.J. Pilling (Leeds), A. R. Ravishankara (NOAA, SPARC co-ordinator for the Laboratory Studies joint project) and T.J. Wallington (Ford Motor Co). After an initial informal discussion in Bilbao, Spain during the Gas Kinetics Symposium, a first workshop took place at the CNRS/Service d’Aéronomie laboratory in University Paris 6, Jussieu, from 7th to 9th December, 1998 to discuss the database. A second workshop from 29th April to 1st May, 1999, in Boulder enabled the evaluation to be finalised and a first draft of a manuscript to be assembled. All the participants have been involved in peroxy radical chemistry, and were in a position to evaluate their own and other studies critically. The aim of the exercise was to formulate a consistent kinetics data set for use in atmospheric chemistry, and to reanalyse data from older studies using these new parameters when necessary to eliminate or reduce systematic uncertainties. The manuscript is now complete, and additionally contains the results of a 3-D model (C. Granier, CNRS-SA, NOAA/CIRES-University of Colorado/ MPI-Hamburg) run with the previously and currently recommended reaction rate coefficients. A summary of the major findings of the evaluation follows.
All the peroxy radicals studied here have absorption spectra in the ultraviolet, below 300 nm. While this absorption does not facilitate photolysis in the lower atmosphere, the spectra are important for laboratory determinations of concentration. Two parameters are necessary for a good description of the spectra — a well-defined shape (absorption as a function of wavelength) and a quantitative measure of the magnitude of the absorption cross section. The novel approach taken in the current evaluation was to evaluate these issues separately — that is because the experimental artefacts that could lead to systematic errors in the two are not necessarily the same. Some of the difficulties encountered include absorption by multiple species, incomplete conversion of starting material into the required radical and an inadequate knowledge of the concentration of radical precursor (which was often chlorine atoms). Furthermore, advances in the understanding of interfering reactions, particularly those between different free radicals, enabled the accuracy of the actinometry to be assessed much more thoroughly than was possible at the time of most of the measurements. All the published spectra were fitted to a common functional form, which enabled outliers to be readily identified, and a best shape to be determined. Then, the experimental conditions were carefully screened to ascertain which absolute determinations were likely to be free of systematic uncertainties, and these data were used to scale the best shape spectrum.
The major goal of this task was to evaluate the current body of kinetic data on peroxy radicals using a consistent set of ultraviolet absorption cross sections. Reanalysis of existing data was performed whenever possible/necessary. Based on the accumulated experience of the panel members, sources of systematic errors were identified and the affected data sets were either corrected or rejected. Data pertaining to the products of each reaction were first evaluated, since an accurate knowledge of the product distribution is often required to derive the kinetic data from the experimental results. The rate coefficients were then evaluated using the recommended absorption cross sections (for absorption techniques) and product distributions. The evaluated rate coefficients are given in Table 1.
Table 1: Summary of Recommended Rate Coefficients
|
|
|
|
|
|
|
| CH3O2 + HO2 | 1 |
5.2 e-12 |
|
|
|
| CH3O2 + CH3O2 | 2 |
3.4 e-13 |
|
|
|
| CH3O2 + NO | 3 |
7.7 e-12 |
|
|
|
| CH3O2 + NO2 | 4 (k0) |
1.3 e-30 |
|
|
|
| 4 (k• ) |
7.6 e-12 |
|
|
||
| CH3O2 + O3 | 5 |
1.0 e-17 |
|
|
|
| C2H5O2 + HO2 | 6 |
7.8 e-12 |
|
|
|
| C2H5O2 + NO | 7 |
8.9 e-12 |
|
|
|
| C2H5O2 + NO2 | 8 (k0) |
4.9 e-30 |
|
|
|
| 8 (k• ) |
7.6 e-12 |
|
|
||
| CH3C(O)O2 + HO2 | 9 |
1.4 e-11 |
|
|
|
| CH3C(O)O2 + CH3O2 | 10 |
1.1 e-11 |
|
|
|
| CH3C(O)O2 + CH3C(O)O2 | 11 |
1.4 e-11 |
|
|
|
| CH3C(O)O2 + NO | 12 |
2.0 e-11 |
|
|
|
| CH3C(O)O2 + NO2 | 13 (k0) |
8.5 e-29 |
|
|
|
| 13 (k• ) |
1.1 e-11 |
|
|
||
| CH3C(O)CH2O2 + HO2 | 14 |
9.0 e-12 |
|
|
|
| CH3C(O)CH2O2 + CH3O2 | 15 |
4.0 e-12 |
|
|
|
| CH3C(O)CH2O2 + NO | 16 |
8.0 e-12 |
|
|
|
| CH3C(O)CH2O2 + NO2 | 17 |
6.4 e-12 (1atm) |
|
|
|
Rate coefficients are given in units of cm3 molecule-1 s-1.
For bimolecular reactions, the temperature dependent expression
is given by k(T) = A exp[-(E/R)/T].
For pressure dependent reactions (4,8,13) the temperature dependent
expressions are k0(T) = A(T/298)-n and k• (T) = A(T/298)-m.
The largest changes (and most discussion) involved the chemistry of the acetyl peroxy radical. This is related to the fact that its reactions lead to a "cascade" of other radicals, all of which contribute to the measured UV absorption and to the observed kinetic decays. The reaction of acetyl peroxy with CH3O2 had one of the highest uncertainties of all those considered. Kinetic data obtained using fairly similar techniques were in tolerable agreement, but the analysis had been conducted using different product distributions. A careful analysis of the available product studies indicated that the majority of the reaction at room temperature proceeds by formation of free radicals, CH3 + CO2 + CH3O + O2. The other channel, which produces acetic acid, CH3C(O)OH, has been suggested as a source of acetic acid in the free troposphere. However, no strong evidence could be found for its occurrence. When the available kinetics studies were analysed using a consistent product distribution, the apparent agreement in the rate coefficients actually worsened! The evaluated rate coefficient, 1.1 x 10-11 cm3 molecule-1 s-1 at 298 K, while thought to be more reliable than previously, is still subject to some uncertainty, and demands further study.
Despite two decades of study, it is clear that more studies are required, particularly at low temperatures. A major recommendation of the evaluation is that new, more specific detection techniques for the study of peroxy radical kinetics be developed. As noted earlier, UV absorption is neither sensitive nor specific, and the results obtained are often ambiguous. Mass spectrometric or high-resolution optical techniques using a different spectral region may be amenable for this goal. Also, use of more diverse ways of producing the radicals would be beneficial. Finally, it is hoped that the evaluation will be used as a solid basis for both the design and analysis of future experiments.
Claire Granier of this group briefly assessed the impact of the evaluated rate coefficients using the IMAGES model, a 3D model developed at NCAR in Boulder. Not all the possible consequences were examined. Figure 3 shows the changes in the calculated concentration of PAN at two altitudes when the rate coefficients recommended in Table 1 are used in place of the values used in current models. This is provided only as an example, and further modelling studies in the future will elucidate the consequences of all the changes noted here.
A detailed paper describing this analysis and evaluation will be submitted to JGR-Atmosphere in June 2000. The database used in this analysis will be available at the SPARC data center in the near future. The availability of this database will be announced in a future newsletter.
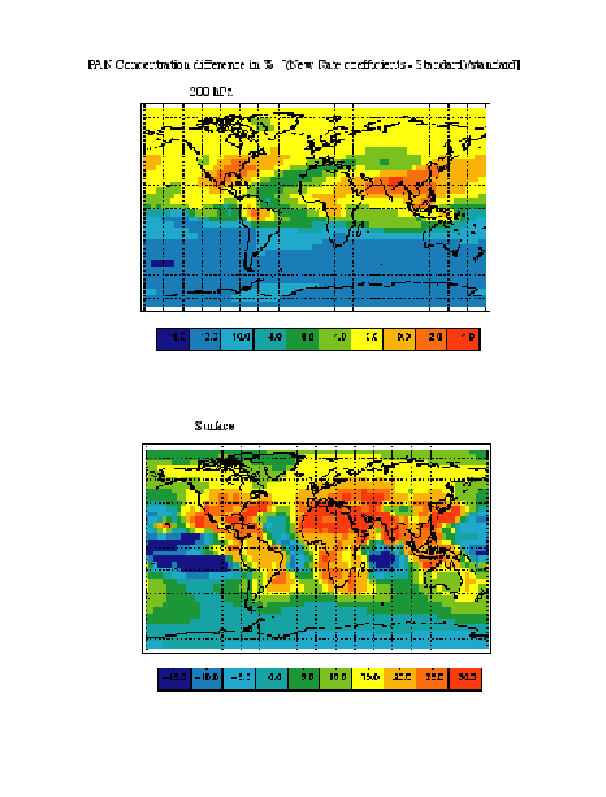
Figure 3. The results of the IMAGES model showing the percentage change in calculated abundance of peroxy acetyl nitrate (PAN) when the rate constants recommended here are used in place of the currently used values. The changes at two altitudes, surface and 200 hPa, are shown. PAN is crucial for the transport of NOx from one region to another and couples the ozone production in one region to another via transport.
![]()