 |
Stratospheric Processes And their Role in Climate
|
||||||||
| Home | Initiatives | Organisation | Publications | Meetings | Acronyms and Abbreviations | Useful Links |
![]()
 |
Stratospheric Processes And their Role in Climate
|
||||||||
| Home | Initiatives | Organisation | Publications | Meetings | Acronyms and Abbreviations | Useful Links |
![]()
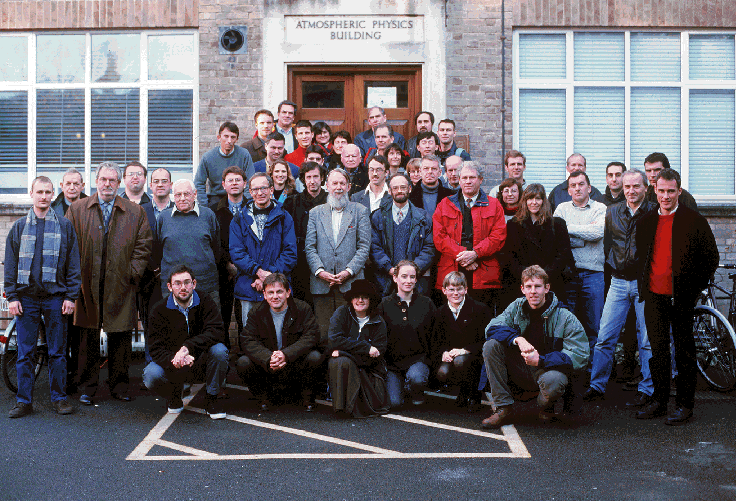
First row: Alan Iw, Steven Pawson, Amanda Kerr-Munslow, Vicky
West, Sarah Sparrow, Maarten van Aalst.
Second row: Ross Bannister, Joe Farman, Adrian Tuck, Kirill Semeniuk,
Darryn Waugh, Alan Brewer, Peter Siegmund, Jim Holton, Emily Shuckburgh,
David Rind, Michael McIntyre, Philip Mote, John Austin, Hans Graf,
Alan Plumb, Lesley Gray, Kristie Boering, John Pyle, Alan O'Neill,
Warwick Norton,.
Third row: Peter Haynes, Dave Stainforth, Michael Palmer, Bill
Grose, Katrin Nissen, Antje Dethof, Martyn Juckes, Bill Randel,
Lon Hood, Giovanni Pitari, Hubert Teyssedre.
Fourth row: Tim Hall, Ulrich Schmidt, Bjorn Hassler, Karen Rosenlof,
Eliza Manzini, Adrian Simmons, Byron Boville, Toshihiko Hirooka,
Kevin Hamilton, John Thuburn.
To mark 50 years since Brewer's seminal paper on the transport of water vapour (Brewer, 1949), and 70 years since Dobson's paper on the transport of ozone (Dobson et al., 1929), a 3-day workshop was held to review all aspects of the Brewer-Dobson circulation. The Subdepartment of Atmospheric, Oceanic and Planetary Physics, University of Oxford, was an appropriate venue since Alan Brewer worked there from 1948 to 1962 and Gordon Dobson worked there from 1920 to 1956 (and continued his research from his house on Shotover Hill on the outskirts of Oxford until his death in 1976).
There were 33 talks given at the workshop which covered topics including the driving mechanisms of the Brewer-Dobson circulation (with particular emphasis on the tropical region), new diagnostics to understand stratospheric transport and mixing, the representation of the Brewer-Dobson circulation in analysis systems and GCMs, diagnosis of the Brewer-Dobson circulation from long-lived tracers (e.g. age of air, water vapour and ozone), and possible future changes in the Brewer-Dobson circulation from changes in greenhouse gases.
The workshop started off with talks by Jim Holton and Alan Brewer of how our understanding of Brewer-Dobson circulation has developed over the last 50 years. Transcriptions of these particularly interesting talks are reproduced here.
One can divide the history of the study of the Brewer-Dobson circulation into 5 decades, more or less. First in the '50s people who thought like physicists were attacking the problem and moved the subject forward through physical reasoning. In the '60s, thanks to the MIT school where I was a graduate student at the time, we really went backwards because of the domination of the Eulerian mean way of thinking. In the '70s, with Andrews and McIntyre and others, theory moved us forward. In the '80s, benefiting from this theory, modelling moved us forward. In the '90s, it was observations with the Upper Atmosphere Research Satellite, and theory done here (in the UK) and elsewhere. The last couple of decades will be covered by other people, so I will concentrate on the first three decades.
So what is the Brewer-Dobson circulation? It is summarised in the classic figure from Brewer (1949). This beautiful diagram has arrows showing circulation upward through the tropical tropopause poleward and then downward, with a concentration showing a stronger circulation in the winter hemisphere than in the summer hemisphere. The numbers mentioned were quite remarkable, Brewer (1949) said `The observed distributions of water vapour can be explained by the existence of a circulation in which air enters the stratosphere at the equator, where it is dried by condensation, travels in the stratosphere to temperate and polar regions, and sinks into the troposphere. The sinking however, will warm the air unless it is cooled by radiation and the idea of a stratosphere in radiative equilibrium must be abandoned. The cooling rate must lie between about 0.1 and 1.1°C per day but a value near 0.5°C per day seems most probable. At the equator the ascending air must be subject to heating by radiation.' It is interesting to see that he didn't say the air is forced to ascend by heating, but rather in order to ascend it had to be heated.
What was Dobson's role? Brewer (1949) quotes from Dobson et al. (1929): 'The only way in which we can reconcile the observed high ozone concentration in the Arctic in spring and the low concentration in the tropics, with the hypothesis that the ozone is formed by the action of the sunlight, would be to suppose a general slow poleward drift in the highest atmosphere with a slow descent of air near the poles. Such a current would carry the ozone formed in low latitudes to the poles and concentrate it there. If this were the case the ozone at the poles would be distributed through a moderate depth of atmosphere while that in low latitudes would all be high up.' So had Dobson figured it out in 1929? Definitely not; Dobson (1929) goes on to say that it couldn't work this way because there was no evidence in 1929 that the altitude of ozone maximum in high latitudes was lower than it was at low latitudes which would be required for that circulation to hold.
Next in Dobson, Brewer and Cwilong (1946) they comment 'It has often been suggested that the difference in temperature in the stratosphere between low and high latitudes is due to general world circulation between the equator and the pole which causes the air in the stratosphere to rise slowly near the equator and subside slowly near the pole.' They (i.e. Dobson) then expressed doubts as to whether this is really the case. However Dobson (1956) on the basis of better ozone observations, Chapman theory, and Brewer's (1949) work had accepted the global circulation in the stratosphere. But, his schematic figure is not as good as that of Brewer (1949). He still thought that vertical mixing by turbulence was a predominant factor.
In 1959 as an undergraduate at Harvard I took my first meteorology course, taught by Richard Goody. Goody told me that we had the necessary information to calculate the heat budget of the stratosphere. He thought this would be a great PhD project for somebody, and indicated that he would like me to stay at Harvard and work with him. Fortunately I didn't take up that topic because soon after that, Murgatroyd and Singleton (1961) published an analysis of the heat budget of the stratosphere. As a result of calculating the heating and cooling rates they calculated what we now call the diabatic circulation. They did point out there were serious problems in the momentum budget, but didn't address this any more than had Brewer or Dobson earlier.
When I started graduate school at MIT in the early '60s, people were aware of the work of Dobson and Brewer, and Murgatroyd and Singleton, but the message that the professors at MIT gave was that this work was all wrong and could be ignored. We lost a lot of time through the '60s because of two different approaches. First there were deductions from radioactive tracers. In the late '50s there was extensive atmospheric nuclear testing by the US in the tropical Pacific. Very early on, Dyer and Yeo (1960) in Australia argued that the pattern of radioactive fallout measured on the ground at Melbourne was consistent with the Brewer-Dobson circulation, with the maximum observed a few months after the equatorial explosion. But Feely and Spar (1960) in the US, basing their information on observations of tungsten 185 in the stratosphere from U2 aircraft, saw rapid meridional transport along the isentropes with very little evidence of an upward and poleward advective circulation. They argued that Brewer and Dobson were wrong, and that really eddy motions were mixing things along isentropes. Reggie Newell at MIT was one of the primary backers of the view that it was meridional mixing by eddies that controlled the distribution of tracers and the heat budget. In Newell (1963) he said `Large scale quasi-horizontal eddies can transport ozone poleward in sufficient quantities to account for the spring build up of ozone. Such large-scale mixing as opposed to mean meridional motions also allows explanation of radioactive tungsten in the stratosphere.' Sawyer (1965) argued that to explain the heat budget you didn't need a mean meridional circulation at all, it could all be done by eddies.
The other line of reasoning in the '60s which took us back a bit was the Victor Starr school of general circulation research; the Eulerian mean approach for understanding the circulation. Vincent (1968) had the most complete analysis of the Eulerian mean meridional circulation in the stratosphere. He found (which had been found somewhat earlier) that if you do averaging on isobaric surfaces around latitude circles that, rather than seeing the single per hemisphere cell Brewer-Dobson circulation, there are two cells, with a reverse cell in the high latitudes with upward motion in the poleward region and downward motion in mid-latitudes.
Discovery of this lead to discussion, when I was a graduate student at MIT, of a magical situation in which the eddy motions were able to work against that reverse Eulerian mean meridional circulation in just the right manner to produce poleward and downward ozone transport, and the poleward water transport.
As far as I can tell, in the early part of the '70s, there was minimal activity in the study of transport in the stratosphere. The next real progress was in the work of Andrews and McIntyre at Cambridge in the introduction of the transformed Eulerian mean (Andrews and McIntyre, 1976) and then the generalised Lagrangian mean (Andrews and McIntyre, 1978). But the transformed Eulerian mean paper wasn't addressing tracer transport but rather the momentum and heat budget of the stratosphere, only later people began using this as a way to approximate the transport circulation.
But before that, right at the end of the '60s, Jerry Mahlman (1969) in a general circulation model study had pointed out that the way you do the averaging really makes a big difference. He found in his GCM that if you did the Eulerian mean averaging, as the MIT school would have you do it, that indeed his GCM showed the two cell circulation with upward motion over the pole and sinking in mid-latitudes. But if he took a scheme which averaged along the streamlines of the jet stream, he then found he had a single cell very much like Brewer had originally shown. That paper didn't have much influence for quite a while, but after Andrews and McIntyre had done the work on the transformed Eulerian mean and the generalised Lagrangian mean, Wallace (1978) and then Matsuno (1980) gave a schematic interpretation to help people understand why the way you average really makes a difference.
Dunkerton (1978), when he was my student at the University of Washington, after reading Andrews and McIntyre, and thinking back to Murgatroyd and Singleton, quickly put a picture of the middle atmospheric circulation together in a paper which used a simplified version of the Andrews and McIntyre Lagrangian mean, together with the heating distribution of Murgatroyd and Singleton. His diagram showed mean transport streamlines with the Brewer-Dobson circulation in the stratosphere and a single pole-to-pole solstice circulation in the mesosphere.
I'll skip forward through the '80s and end with a couple of comments on the '90s. With the work of Haynes et al. (1991), we now have a much better understanding of what is forcing the Brewer-Dobson circulation, which is the upward propagation of planetary and gravity waves from the troposphere. Wave breaking, particularly planetary wave breaking in the winter stratosphere, produces this westward force which McIntyre gave the name `wave driven pump'. Because the earth is rapidly rotating, you get a quasi-gyroscopic effect in which a westward force produces a poleward drift. The poleward drift will draw air up in the equatorial region, which will then become colder than radiative equilibrium and so becomes radiatively heated, and push air down at high latitudes accompanied by radiative cooling. Here is the Brewer-Dobson circulation. The momentum budget problem, which was so difficult to understand for Brewer, Dobson, Murgatroyd and Singleton, is now solved by this wave breaking idea.
This same circulation will transport tracers. A vertically stratified tracer will tend to have its mixing ratio surfaces tilted by the effect of the upward and downward motion, but at the same time, the wave breaking is producing mixing in mid-latitudes. The effect of that mixing is to tend to homogenise the tracer distribution in this region. So that a typical mixing ratio surface will end up looking something like this, lifted high in the tropics, depressed downward in the polar vortex, and with a more or less level surface in mid-latitudes. That is just the sort of thing we see from the wonderful data from the UARS satellite, for example methane as viewed by the HALOE instrument.
The Brewer-Dobson circulation has recently become the standard way in which one tries to explain and understand the global-scale exchange between the troposphere and the stratosphere. This is taking us back in 1999 to 1949 where Brewer was explaining stratosphere/troposphere exchange of water vapour, which is one of the primary problems we are still working on today.
Andrews, D. G., and M. E. McIntyre, Planetary waves in horizontal and vertical shear: the generalised Eliassen-Palm relation and the mean zonal acceleration, J. Atmos. Sci., 33, 2031-2048, 1976.
Andrews, D. G., and M. E. McIntyre, Generalised Eliassen-Palm and Charney-Drazin theorems for waves on axisymmetric mean flows in compressible atmospheres, J. Atmos. Sci., 35, 175-185, 1978.
Brewer, A. W., Evidence for a world circulation provided by the measurements of helium and water vapour distribution in the stratosphere, Quart. J. Roy. Meteor. Soc., 75, 351-363, 1949.
Dobson, G. M. B., A. W. Brewer and B. Cwilong, Meteorology of the lower stratosphere, Proc. Roy. Soc., Series A, 185, 144-175, 1946.
Dobson, G. M. B., D. N. Harrison and J. Lawrence, Measurements of the amount of ozone in the earth's atmosphere and its relation to other geophysical conditions, Proc. Roy. Soc., Series A, 122, 456-486, 1929.
Dobson, G. M. B., Origin and distribution of polyatomic molecules in the atmosphere, Proc. Roy. Soc. London, A236, 187-193, 1956.
Dunkerton, T. J., On the mean meridional mass motions of the stratosphere and mesosphere, J. Atmos. Sci., 35, 2325-2333, 1978.
Dyer, A. J., and S.-A. Yeo, A radio-active fallout study at Melbourne, Australia, Tellus, 12, 195-199, 1960. Feely, H. W. and J. Spar, Tungsten-185 from nuclear bomb tests as a tracer for stratospheric meteorology, Nature, 188, 1062-1064, 1960.
Haynes, P. H., C. J. Marks, M. E. McIntyre, T. G. Shepherd and K. P. Shine, On the "downward control" of extratropical diabatic circulations by eddy-induced mean zonal forces, J. Atmos. Sci., 48, 651-678, 1991.
Mahlman, J. D., Long-term dependence of surface fallout fluctuations upon tropopause-level cyclogenesis, Arch. Met. Geophys. Bioklim., A 18, 299-311, 1969.
Matsuno, T., Lagrangian motion of air parcels in the stratosphere in the presence of planetary waves, PAGEOPH, 118, 189-216, 1980.
Murgatroyd, R. J. and F. Singleton, Possible meridional circulations in the stratosphere and mesosphere, Q. J. R. Meteorol. Soc., 87, 125-, 1961.
Newell, R. E., Transfer through the tropopause and within the stratosphere, Quart. J. Roy. Meteor. Soc., 89, 167-204, 1963.
Sawyer, J. S., The dynamical problems of the lower stratosphere, Quart. J. Roy. Meteor. Soc., 921, 407-416, 1965.
Vincent, D. G., Mean meridional circulations in the Northern Hemisphere lower stratosphere during 1964 and 1965., Quart. J. Roy. Meteor. Soc., 94, 333-349, 1968.
Wallace, J. M., Trajectory slopes, countergradient heat fluxes and mixing by lower stratospheric waves, J. Atmos. Sci., 35, 554-558, 1978.
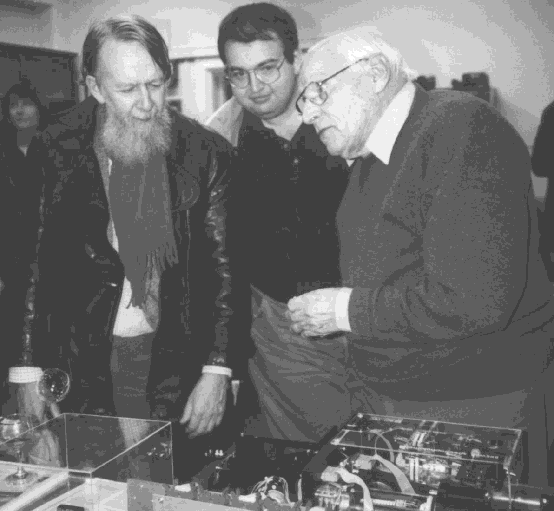
Alan Brewer, Kirill Semeniuk, and Michael McIntyre discussing the Brewer Spectrophotometer
My involvement with the stratosphere began in the height of summer 1942, in the very depths of World War II. The Germans controlled the Atlantic coast, from the North of Norway to the south of France. They were besieging Stalingrad, in sight of Moscow, crossing the Donne and looking forward to dealing with the great oil fields of Asia Minor. In Africa they had reached El Alamein and Rommell was satisfied that with one last push they would capture Alexandria, the Nile Valley and the Suez canal. The Commonwealth would be split in two and Germany would join with Japan.
Me, I was just a shift forecaster at an RAF station not far from Oxford. In an afternoon off, a messenger came to say I had to return to the office because I was going to be moved. I was informed that I had to be at HQ in London first thing the next day. I got there and was briefed. The problem I was asked to deal with was condensation trails. It is quite easy to make a physical theory in which you balance the effects of water and heat from the aircraft. In a height-temperature plot, on one side of a line that we called Mintra (sloping from high altitude and low temperature to low altitude and high temperature) an aircraft was able to make trails because its passage increased the humidity, on the other side it was not. Everybody was very satisfied with this theory and this is what the pilots were told. However, in practice, pilots were rather worryingly reporting trails when they were flying at moderate altitude in regions where we expected no trails; and they were reporting no trails when flying at high altitude in regions where we were expecting big trails. Understanding this discrepancy was very important. In a raid on Nuremberg we had lost 93 aircraft, a totally unsustainable loss. It was therefore essential that we found out what was happening.
I was invited to investigate this. I was sent to Boscombe Down to a special unit, which was to be just me, attached to High Altitude Flight. I was briefed by the Director of the Met Office himself, who emphasised the problems and also mentioned the difficulties of making air temperature measurements from aircraft. I was told that Dobson had already started on hygrometry and would help and supervise. I was to have 2 Bostons and 1 sergeant instrument maker. (When I arrived at High Altitude Flight, I met a recent Cambridge graduate, a red-headed character who proved immensely helpful. His name was Richard Goody, and he was concerned with the avionics section. We worked side-by-side for the rest of the war.)
I realised that the air temperature measurement problem was a very acute one, and I soon learned that no-one had any idea how to solve it. (They were very busy, let me say). I knew that there was a real problem because we were getting quite ridiculous temperature measurements across the forecast bench. So the first thing to do was to solve this problem, which I was able to do very quickly and with great asset to my own prestige. A forecaster's prestige was not really very good -in modern terms somewhere between a lawyer and a politician- and to come in and solve a problem that was worrying the experts was a good start and it stood me in good stead for a long time. It also enabled me to capture a very competent instrument maker, whom Richard Goody had tipped me off about.
Dobson was rather annoyed at the delay. Dobson looked after me very closely, travelling the 50 miles down from Oxford to Boscombe Down. In the first place Dobson supplied a hygrometer which he had shown to work in the lab. under a wide range of temperatures. However, in the aircraft it suffered from the fact that the light changes as the aircraft passes through cloud and turns etc. So, for aircraft use it was clear that we needed a proper illumination system. We therefore further developed the frost point hygrometer. The object is to watch for deposition of dew or frost on a surface that is ventilated by outside air. It is cooled by pumping a coolant from below. The temperature is measured by a resistance thermometer. The viewing surface is at a focus of an elliptical glass lens, with a lamp at the other focus for illumination. You watch for deposition on the surface through a good magnifying glass. We soon had this working and the first time we made condensation trails under conditions when we ought not to have made them, it was clear that we were in that no-man's land where the air was saturated with respect to ice, but was not saturated with respect to super-cooled water; so the air could be clear, but the passage of the aircraft caused the trail. We had that solved fairly quickly. There was not much trouble with the fact because the physical possibility was well known.
The Boston aircraft had a performance almost exactly equal to that of the modern Hercules, with a ceiling of about 30,000 ft and so we could not get into the stratosphere where the expected trails did not occur. The air force provided a Flying Fortress said to be one of six that were given as a personal present from Roosevelt to Churchill. We took everything we could move out of it and we got it to 37,000 ft and this was the first time we got into the stratosphere. To my surprise, as the temperature turned up, the frost point turned down, and at the highest levels I could get no deposit. We were using solid carbon dioxide as a coolant and we obviously had to change to liquid oxygen, which we did, but it was clear that the reason that there were no trails in the stratosphere was that the air was exceedingly dry. I would not have believed before I had started that the air could be so dry, but I saw it with my own eyes. I had plenty of trouble with people convincing them that we could really measure that dryness.
The war progressed and we got the Mosquito aircraft. The instrument was redesigned and rebuilt to work in the pressure cabin. The Mosquito was a lovely airplane. The cockpit was size of a small car and I sat side-by-side with the pilot, with the frost point hygrometer by my right hand and a short pipe going outside. I made very great effort indeed to ensure that the exceedingly dry measurements were really right. With the Mosquito could get higher, and with the new instrument the lowest frost point I ever measured was 190K absolute. The effects at the tropopause were very striking. Personally, I very soon formed the opinion that the very dry air had come from the equatorial tropopause. All we knew about temperatures at different altitudes in the tropics came from Brunt (1934), which had a figure based on measurements in the Dutch East Indies, now Indonesia. This showed the equatorial tropopause to be about 190K absolute consistent with my lowest frost point.
Dobson gave the 1946 Bakerian Lecture (Dobson, 1946). It was Dobson's lecture; Cwilong's name and my name were put on it simply because he used the humidity measurements. If you examine it you will find that he is actually merely reviewing the traditional theory that had been started by Emden (1913) and had continued through Simpson (1928) and in it he recalculates the radiation balance of the stratosphere with the new humidity measurements. Simpson had assumed that there was 0.3mm of precipitable water in the stratosphere, we had divided that by something like 50. Dobson was attempting to make a new calculation with the new concept of the dryness.
Eric Eady was an old friend. We had been on a Met Office training course together, we had worked on the same forecast bench. He came and visited me occasionally and he would stay at my home. He came to my office and we would talk. He would tell me about long waves in the westerlies and baroclinic waves and I would tell him how the stratospheric air had come from the equatorial tropopause. He was not convinced. He had very great difficulties with the momentum problem and in fact there is a caveat in my 1949 paper (Brewer, 1949) to the effect that there is a problem with momentum. Dobson was not very impressed with my suggestions as an explanation of the ozone observations. I came to Oxford and at about that time Dobson began to work with R.H. Kay developing a sampler to measure ozone concentration that could be flown on an aircraft, it was based around being flown on a Met Flight Mosquito. It followed the method in which iodine is produced by the ozone and is measured electrochemically. We got no useful results in Southern England. Then one day Dobby came into the lab and said he had had a letter from Tönsberg in Tromsö. Tönsberg was the very active director of the observatory in Tromsö and had worked with Dobson for many years on ozone measurements. The early high latitude measurements that are so often quoted are due to him. I suspect that Tönsberg had been measuring the low level of the ozone and no-one was believing him. Tönsberg said that if we sent someone with the sampler he could get flights from Tromsö to measure ozone through the tropopause. Dobby asked me what we should do and I said we should take up the offer. Dobby asked who we should send and I said a student, and if we can't find a student then I would go. Well, we didn't have a student and I went. I was able to get 4 good flights there in July 1955 on a Vampire jet that flew to 43,000 ft. The results showed a very sharp transition in ozone concentration at the tropopause (Brewer, 1957). Dobby had been totally committed to a stagnant stratosphere, but after these results he was convinced and he published his well known paper. The results convinced me that we needed an ozonesonde. Soon afterwards James Milford joined the department as a graduate student and took on the job of making one.
Now, there was in the late fifties the atom bombs and hydrogen bombs. The hydrogen bombs were penetrating stratosphere and going to a great height. We were told that it didn't matter because the debris would go up there and float around for years and years, quietly decaying. But, of course it came out PDQ. What was more, they fired the hydrogen bombs in the Pacific, but the debris came out in a broad swathe from California to Boston, where it worried people who mattered. As a result, in New York in 1960 the UN Scientific committee held a discussion on the problem of the transfer of debris into the troposphere. I received a letter from the Director of the Met Office, who was Sutton at the time, saying that he had an invitation for me to go to the discussion, but that there were no provisions in any budget for my expenses. So, I proposed not to go, but I was on sabbatical leave at MIT at the time, and MIT encouraged me to go and offered to pay my expenses. So I went. It was a meeting of about 50 people, and it covered a lot more than just the stratosphere, focusing on all aspects of the fallout problem. I gave my opinions and MIT, having paid my expenses, reasonably invited me to write up what I said. I had forgotten about this until a copy of the MIT report (Brewer, 1960) turned up in Dobson's papers and was brought to my attention by John Barnett. One of the things that I pointed out was that there is a great readjustment of the level of ozone. We had made some ozone ascents in 1958 using Milford's ozonesonde. Some were made in Antarctica, I went to Malta (36°N), and I sent John Houghton (!) to Tromsö (70°N). The differences at different latitudes were very clear. In my 1949 paper I noted that there has to be this circulation, but I hadn't the foggiest how fast it was. It might be a hundred metres a day, it might only be a few. By 1960, it was obviously fairly large because the fallout was coming very fast. I guessed, based on the outflow of radioactive debris, that the annual downflow was a tenth to a fifth of the hemispheric store. This was the greatest I dared say without being laughed out of court.
While I was at MIT on sabbatical in 1960 I had a bet with Reggie Newell. At the time, I said that I bet 25 cents that in spring we would get new outflow events even though there had been no bomb in the last six months. He bet 25 cents that there wouldn't be. We wrote it on a piece of paper, got it properly typed and signed it. I found the piece of paper about 20 years later and sent it to Reggie, claiming my 25 cents with interest. He sent me a silver dollar.
In 1960, Anthony Wilson joined the department and I invited him to make measurements of the UV that was producing the ozone. The hope was that by measuring the UV radiation at wavelength 200nm and its divergence we could, by using the Chapman equations, get an idea of the rate of ozone production and perhaps the rate at which it was being turned over. The results that we published in 1965 were that the solar radiation at wavelength 200nm is three times less than that reported by Detwiler et al. and that the oxygen absorption cross-section is about 25% lower than reported in Ditchborne and Young. We went on to examine what the consequences of this were. I thought that if we took an account of the whole global store of ozone and watch it swilling about from the equator to the winter hemisphere, and assume that it is always going downwards (so that it is made but never comes back), we ought to get a rough idea of the total turnover in ozone. We did the calculation and found that the downward transport was much larger than I had expected with about half the total store of ozone coming down each year. There was no doubt that the turnover was very large, and it was confirmed by the debris data that was coming in. The calculation also showed that the outflow is seasonal and that in the northern hemisphere is about three times that in the southern hemisphere. So then we set out to do the calculations to relate the radiation to the outflow of ozone. One problem is, where is the ozone being made? This was discussed in our 1968 paper (Brewer and Wilson, 1968). The difficulty was to get people to grasp that the ozone that matters is not being made at the top of the atmosphere, it is being made relatively low down. At the top of the atmosphere the ozone concentration, in mixing ratio terms, is highest and therefore people thought that this is where the ozone is made, but in fact this region is in photochemical equilibrium. This means that the Lagrangian equation gives you no ozone production, there may be lots of chemistry going on, but there is nothing being made to transport downwards. The main production region is at 10mb. There is considerable negative feedback in the ozone production process. If at high levels, some of the ozone is destroyed by nitrogen dioxide or chlorine, then the radiation that was absorbed by that ozone is able to penetrate to lower levels where it is able to create more ozone. This gives considerable stability to the ozone levels in the world.
Much the same story was told from radioactive fallout. The radioactive fallout is a body of very useful information and I hope that people are aware of it and will make use of it where possible. In a figure from Cambray et al., quoted by Peirson, (1970), it can be seen from the radioactive measurements that the northern hemisphere fallout is much more than the southern hemisphere fallout, in a ratio of about 3:1, as we deduced from the ozone measurements. In the period around 1966, the debris was pretty uniformly mixed through the stratosphere and the fallout is indeed diminishing by about 50-60 per cent per year. I hope that people realise that this information is available.
What were people thinking around 1970? In 1969 the Royal Meteorological Society (RMS) held a major meeting on discussions of the global circulation. This is an extract from the paper on The structure and dynamics of the stratosphere (Murgatroyd, 1970); `The earliest explanation of the general form of the vertical temperature profile by such workers as Gold (1909), Emden (1913) and Gowen (1947) was that the troposphere is broadly in convective equilibrium and the stratosphere is in radiative equilibrium. These conclusions have been broadly confirmed by recent authors (e.g. Goody 1949, Leovy 1964, Manabe et al., 61,64,67)…’ I would like to say about the 1949 paper that I knew very well that it was not in accordance with most people's views, and that it would be difficult to get published. Indeed, part of the paper was designed to be acceptable to the referees and the editor. It would have been very easy for the referees to rubbish it, and to say that I had no experience in this field, was out of touch and that the idea was ridiculous because it was widely recognised that the stratosphere was in radiative equilibrium. I thank Reggie Sutcliff, who was the editor at the time, for it being published. Twenty years later, with a good deal of hindsight, the RMS paper was written. At this time the humidity measurements were beginning to be accepted, and I've talked about the fallout information, so the conclusions of the paper are particularly surprising.
We are now up to 1970, and I had learned that there was a lot of valuable information in the ozone measurements, but the Dobson spectrometer was becoming a problem. The supply had dried up and it was difficult instrument to maintain and required considerable skill to operate. So, I decided to prepare the ground for a new instrument which was to make use of digital methods of measurement. We had started that work when another stratospheric problem reared its ugly head. Concorde was being built and looked like being a success, and it looked, moreover, that it would become a big European symbol. It was receiving a lot of publicity. The Russians entered the race, making their copy which people called Concordski. The proposal was to build 250 Concordes and 250 Concordskis. The Americans were not to be left out. Boeing had plans for a bigger and better supersonic transport and they were going to make 500. An unthinkable fraction of the world's total fuel resources would be burnt in the stratosphere. I wasn't worried about the stratosphere, but I think there is a lot to be said for walking and cycling!
The suggestion which started to receive a lot of publicity was that NOx would be produced by these transports and is incompatible with ozone, that it would catalytically destroy ozone and in the stagnant stratosphere this NOx would accumulate indefinitely, the ozone would go and we would all die. From what I could see of pollution operations and measurements, NOx and ozone seemed to be positively commensal and the argument that they were mutually destructive I did not think very sensible. Also, was the pristine stratosphere really free of NOx? I wondered whether we could settle something about this by remote sensing method which we were working on for the new Dobson spectrometer. We dug out the spectrum of nitrogen dioxide, and at 450nm (in the blue) there was a very striking feature which meant that we could measure the NO2 between us and the sun. There were three wavelengths quite close together, which gave us a strong curvature of absorption and using methods similar to the Dobson AD method of calculation we could get the NO2 with very little interference from other substances. So we made a suitable instrument and took it onto the roof of the physics building in Toronto, where I was at the time. It was quite obvious as soon as we started that the NO2 in the Toronto atmosphere meant that it was quite impossible to work there. The instrument was quite small. It was considerably smaller than the modern `Brewer' as they call it. The actual spectrograph went in a light-tight enclosure about the size of a top hat, and looked a bit like one too. I decided to take it to the family cottage, our datcha. There, 100 miles at least from either Toronto or Montreal and almost that from Ottawa and Rochester and lots of countryside and Lake Ontario around, we would be free from pollution. There was a very good horizon as the astronomers call it. During the winter of 1972/3 we made a new instrument taking advantage of all the experience we had so far and it was a very considerable success. Part of the requirement for the Dobson successor was transportability and at weekends and holidays I would dump this thing in the car and drive out to the cottage. So we only got measurements on fine days when I was at the cottage at weekends and in the vacations.
The instruments worked liked a dream. It was a fabulous success, it proved very stable and gave consistent results throughout. It was small, so when we felt we wanted measurements from an aircraft, we could use it as a carry-on device, and we could take it from the cabin of the aircraft looking at the sun through the porthole and we could carry it into the pilot's cockpit and take the zenith sky measurement where there was possibility of seeing the sky. We took the measurements in spring and summer 1973 and we decided we needed some high level measurements because the tropospheric pollution is in the way and is very variable. We got some results of the concentration of nitrogen dioxide over southern Ontario. They surprised us very much and I think most people would disagree with them. The striking thing is the very large diurnal variation which we observed. The sunrise and sunset values were remarkably stable throughout the period I was making measurements. We were surprised they were so steady when all the other daytime measurements were so variable, and I understand that in fact they show remarkable little variation over the whole globe. They do vary with latitude and season, but not a great deal. Sunrise is always less than sunset, and the noon is very, very variable. I published the results in '73 in a paper with Kerr and McElroy (Brewer et al. 1973). The sunrise and sunset figures are quite easily measured by a variety of methods, and are accepted. The noon values are totally rubbished.
The Concorde story proved different from what was expected. A Concordski crashed at the Paris Air Show and the Russians abandoned civilian supersonic entirely. After a lot of effort, Boeing decided that their aircraft wasn't even going to get off the drawing board and they abandoned it. So an important prestige field was left to Concorde. This resulted in a very unholy collaboration between Boeing who did not want Concorde to work, and the Greens who did not want anything to work. I am satisfied that we bounced the eco-warriors and got our Nature paper published before they even knew it was there, and it caused great annoyance - still does.
The students that I was working with, Kerr and McElroy, are still interested in the problem and they recently sent me some NO2 measurements taken in the northern suburbs of Toronto. They are from two days close together, and yet they show enormously different mid-day peaks. In interpreting these values you need to take into account that the tropospheric values are highly variable, but I am sure the stratospheric values are variable too. However, there is immensely little data on this. Adrian Tuck (Tuck, 1996), in his very valuable Symons memorial lecture makes no mention of NOx or of NO2, which is rather strange given the amount of fuss that was made about it.
In our 1973 paper there is an example of the observations as they were taken from the instrument. When I look back at it, I see that on 23 July 1973 I started work with the sun 6° below the horizon and was still working when it was 6° below the horizon in the evening. Whether it was a weekend or holiday I don't know. The difference between the morning and evening is visible and when the sun is getting high, at 76° zenith angle, the difference is very clear. The problem is how much is tropospheric and how much is stratospheric.
In the paper we mention the problem of where does the NO2 go at night. We suggested that it turns into NO3. If you put NO2 and ozone in a bottle in the lab it turns a beautiful green with NO3, which has a very nice absorption spectrum. Mervyn Davis came to work with us and made use of this absorption spectrum to measure NO3. We found none. Modern theories include the conversion to N2O5, and N2O5 necessarily includes NO3 on the way. If there is no NO3, or very little, then I don't think that there is much chance of there ever being much N2O5.
I thought a very great deal about this work recently. I came to the conclusion that my measurements were correct. I am totally satisfied in my own mind that in the stratosphere we get a strong but very variable diurnal variation of the NO2 content. We may show its nature by plotting the NO2 against solar elevation (positive and negative). We get a curve rather like the well known hysteresis curve of magnetism. In electronics we sometimes get a similar figure when we produce a Lissajous' figure on an oscilloscope. I think that NO2 follows this sort of variation but differently every day, and that NOx offers the potential of being a very useful tracer.
If we are to get any one to accept this we need a coherent explanation of the chemistry. Contemporary accepted chemistry of NOx, so far as I understand it, seems to me to be very clever; but the effects I observed were very strong and we need a good simple explanation. What does it take to get such a curve? It needs (1) positive feedback (2) storage and (3) nonlinearity provided by limits or stops.
I think there is a simple theory. I suggest that the NOx in the stratosphere has no significant components other than NO and NO2, that the total (NO + NO2) is very variable, and might sometimes be as much as 20 DU. Now it was part of the doctrine of the eco-warriors that there was little NOx in the native, pristine, stratosphere. I think it is more probable that there was (and is) a lot. Stratospheric air comes from the cold equatorial tropopause where thunderstorms are important; and we know that thunderstorms produce a lot if NO. NO will also be produced by lightening between thunderstorm tops and the ionosphere.
NO2, which we measured, is decomposed (photolysed) by radiation from the sun (l < 300nm). These penetrate the atmosphere and always tend to convert NO2 into NO. Why then do we observed the NO2 when the sun is high? It need not be complicated chemistry.
The NO2 occurs in the day through a reaction with atomic oxygen which is being produced by solar UV. It could be through a three body reaction or through a reaction where the excess energy is got rid of by a yellow-green chemiluminesence. This reaction has been shown in the laboratory to occur over a wide range of temperatures and pressures by Gaydon (1946). The suggestion is not entirely stupid. The atomic oxygen is provided by photodecomposition of ozone and also oxygen, it depends totally on the illumination which of course changes from none to full sunlight, and also on the ozone concentrations. Ozone always decompose when it absorbs light. Visible light at 600nm will give a small amount of product so visible light will never get rid of the NO2 completely. At 300-350nm there is strong absorption and rapid production of O when the sun is high, but as the sun goes down you get very much reduced production. There is also production at 200nm from oxygen. This is the atomic oxygen coming from ozone which produces ozone. If the hypothesis that NO2 results from the reaction with atomic oxygen and nitric oxide is right, and if nitric oxide is also very variable, then you have a very complicated picture and it is not surprising that the NO2 is very variable. So I put it forward as a suggestion that I think that NOx, which is not discussed by Adrian Tuck in his Symon's memorial lecture, ought to be discussed, and that you need to consider NO + NO2, nothing more clever. I think there is a lot of useful information there.
Well now it seemed to me that there was a lot of harm being done because NOx was not getting the attention it should and that it was being treated too much as something we knew everything about. So I put this together in a short letter to the editor of the Quarterly Journal. I was not optimistic that it would get accepted; just as I thought I was lucky to get the '49 paper published. But the referees said that I was out of touch. I think that it is very serious that these ideas are too easily thrown out. I can't do anything about this matter now, but clearly much more research on NOx is needed.
Brewer A. W. Evidence for a world circulation provided by the measurements of helium and water vapour distribution in the stratosphere, Q..J. Roy. Meteorol. Soc., 75, 351-363, 1949.
Brewer, A. W., Ozone concentration measurements from an aircraft in N. Norway, Q. J. R. Meteorol. Soc., 83, 266-268, 1957.
Brewer, A. W., Transfer of ozone into the troposphere, M.I.T. Planetary Circulations Project Report, 1960.
Brewer, A. W., and A. W. Wilson, Measurement of solar ultra violet radiation, Q. J. R. Meteorol. Soc., 91, 351-363, 1965.
Brewer, A. W., and A. W. Wilson, The regions of formation of atmospheric ozone, Q. J. R. Meteorol. Soc., 94, 249-263, 1968.
Brewer, A. W., C. T. McElroy, and J. B. Kerr, Nitrogen dioxide concentrations in the atmosphere, Nature, 246, 129-133, 1973.
Brunt, D., Physical and dynamical meteorology, C.U.P., Cambridge, U.K., 1934.
Murgatroyd, R. J., The global circulation of the atmosphere, Ed. G.A. Corby, Roy. Meteorol. Soc., London, p165, 1970.
Dobson, G. M. B., A.W. Brewer, and B.M. Cwilong, Meteorology of the lower stratosphere, Proc. Roy. Soc. A, 185, 144-175, 1946.
Emden, R., Sitzber, Matl-Phys Kl. Akad. Wiss. Munchen, 1913.
Gaydon, A.G., Continuous spectra in flames: the role of atomic oxygen in combustion, Proc. Roy. Soc. A, 183, 101-123, 1943.
Peirson, D. M., World wide deposition of long-lived fission products from nuclear explosions, Nature, 234, 144-175, 1971.
Simpson, C. G., Further studies in terrestrial radiation, Memoirs R. Meteorol. Soc. 3, No 21, 1928.
Tuck, A. F., et al., Symon's Memorial Lecture 1995, Q. J. R. Meteorol. Soc., 123, 1-70, 1997.
![]()